Abstract
Introduction:
Over the past decades ex vivo T-cell depletion via graft engineering (GE) demonstrated its efficacy in reducing the incidence of severe GVHD. To subsequently facilitate immune reconstitution (IR) and prevent or treat relapse of neoplastic diseases after allografting, pre-emptive, prophylactic and curative donor lymphocyte infusion (DLI) is routinely applied in many centers. Although multiple prospective clinical studies have been reported on graft and DLI engineering (for review see [1]), there is no consensus nor harmonization in the use of cell engineering strategies and activity reports on graft and DLI engineering and reimbursement strategies are lacking. We therefore conducted a field survey within the EBMT to obtain an overview of the type of graft engineering, the indication for, and reimbursement of GE and modified DLI.
Methods:
The EBMT Cellular Therapy & Immunobiology Working Party (CTIWP) conducted a survey among EBMT centers to identify centers performing graft and/or DLI engineering between January 2011 and December 2020. With regards to GE, frequencies of CD34 selection, αβTCR depletion, αβTCR/CD19 depletion, CD3 depletion, CD3/CD19 depletion and CD45RA depletion were obtained. With regards to modified DLI, frequencies of Treg depletion, CD45RA depletion, administration of antigen specific T cells or other types of DLI modification were obtained. Descriptive statistics were utilized to analyze answers by centers participating to the survey, as well as distribution of responses according to the questionnaire.
Results:
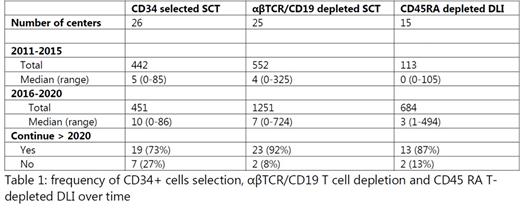
Responses were received from 115 participating EBMT centers active in 30 countries. 36% (n=41) of the responding centers performed GE (adults n=10; pediatrics n=18; adults&pediatrics n=13). Within the centers performing GE, in 68% GE was applied for both malignant and non-malignant indications. 85% of the centers utilized GE for haplo-identical donors, but also for other donor types (identical sibling 34%; 10/10 MUD 49%; 9/10 MUD 42%) GE was performed. Within the different modalities of GE, CD34 selection (63%) and αβTCR/CD19 depletion (61%) were most frequently applied, with a variable number of procedures per center (Table 1). For CD34 selection the total number of SCT procedures was stable within 2011-2020 (442 from 2011-2015; 451 from 2016-2020), whereas for αβTCR/CD19 depletion there was a marked increase (552 from 2011-2015; 1251 from 2016-2020). There might be a tendency that fewer centers continue with CD34 selection after 2020 (73%), whereas for αβTCR/CD19 depletion 92% of the centers continue after 2020 (Table 1). Other types of GE such as CD3/CD19 depletion and 'Campath in the bag’ were practiced in fewer centers participating in this survey (32% and 10% respectively). 71% of the centers applied more than one form of GE. 73% of the centers performed GE solely as routine practice and 22% of the centers performed GE both for routine practice as well as clinical trials. 59% of the centers received reimbursement for GE procedures from the health authorities.
We also inventoried the local practice of DLI. 21% (n=24) of the responding centers reported implementation of modified DLI, with CD45RA depletion (63%) being most frequently applied. The number of administered CD45RA depleted DLI increased over time (113 from 2011-2015; 684 from 2016-2020).
Conclusion:
Graft and DLI engineering is increasingly used within EBMT centers between 2011 and 2020, and is dominated by αβTCR/CD19 depletion (n=1803) and CD45RA depleted DLI (n=797). Retrospective analyses of this large dataset will allow to assess long-term clinical outcomes and compare GE and modified DLI to further develop such strategies within the context of other low-GVHD allo-HSCT platforms and 'post-allo interventions'.
1. de Witte, M., et al., Allogeneic Stem Cell Transplantation Platforms With Ex Vivo and In Vivo Immune Manipulations: Count and Adjust. Hemasphere, 2021. 5(6): p. e580.
Disclosures
Chabannon:BMS/CELGENE: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; JANSSEN PHARMACEUTICALS: Membership on an entity's Board of Directors or advisory committees; EBMT: Membership on an entity's Board of Directors or advisory committees; MILTENYI BIOTECH: Research Funding; TERUMO BCT: Speakers Bureau; FRESENIUS KABI: Research Funding; BELLICUM PHARMACEUTICALS: Membership on an entity's Board of Directors or advisory committees; GILEAD: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; NOVARTIS: Speakers Bureau; SANOFI SA: Honoraria, Research Funding, Speakers Bureau. Malard:Therakos/Mallinckrodt: Honoraria; Sanofi: Honoraria; Astellas: Honoraria; JAZZ pharmaceuticals: Honoraria; Biocodex: Honoraria; Janssen: Honoraria; Takeda: Honoraria; Novartis: Honoraria; Gilead: Honoraria; Celgene-BMS: Honoraria. Kuball:Novartis: Research Funding; Miltenyi Biotech: Patents & Royalties: novel CAR T and engineering isolation strategies, Research Funding; GADETA: Current equity holder in private company, Patents & Royalties: on gdTCR engineering strategies and targets , Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal